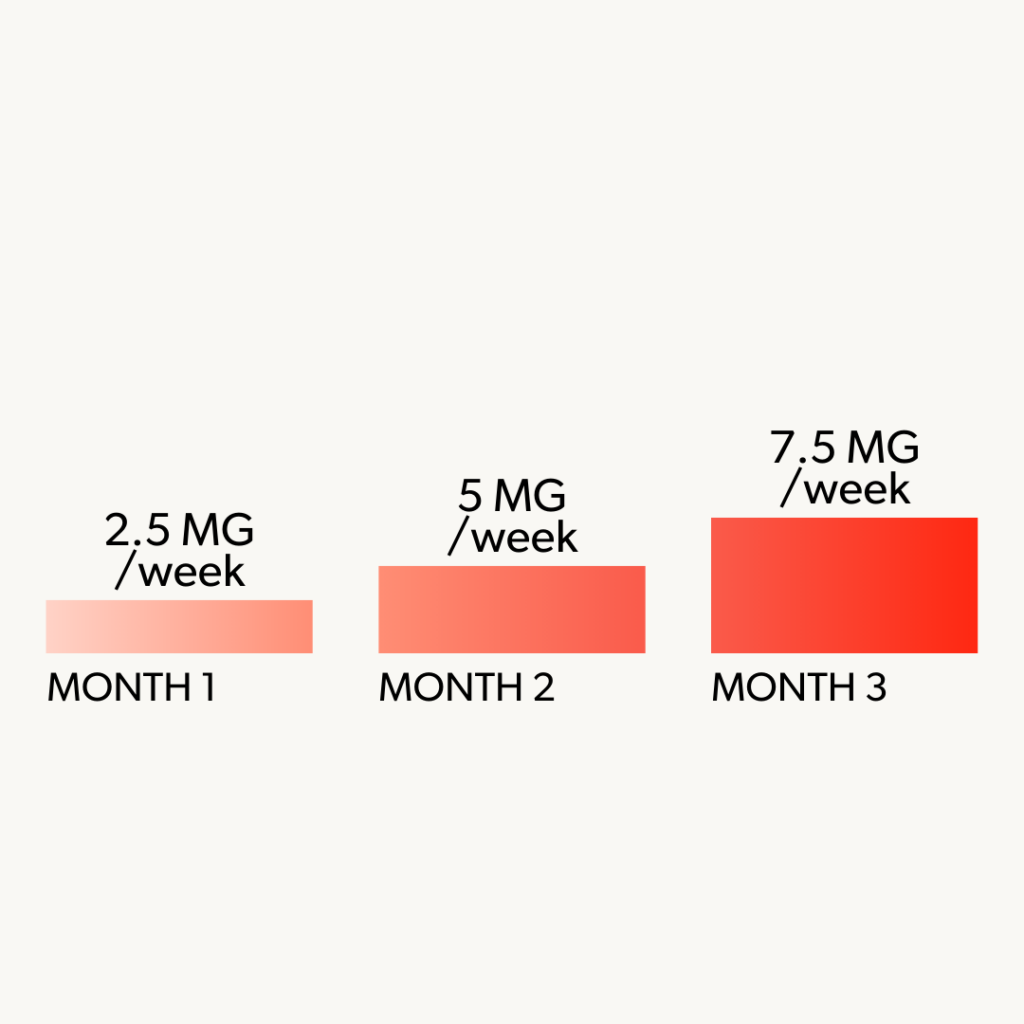
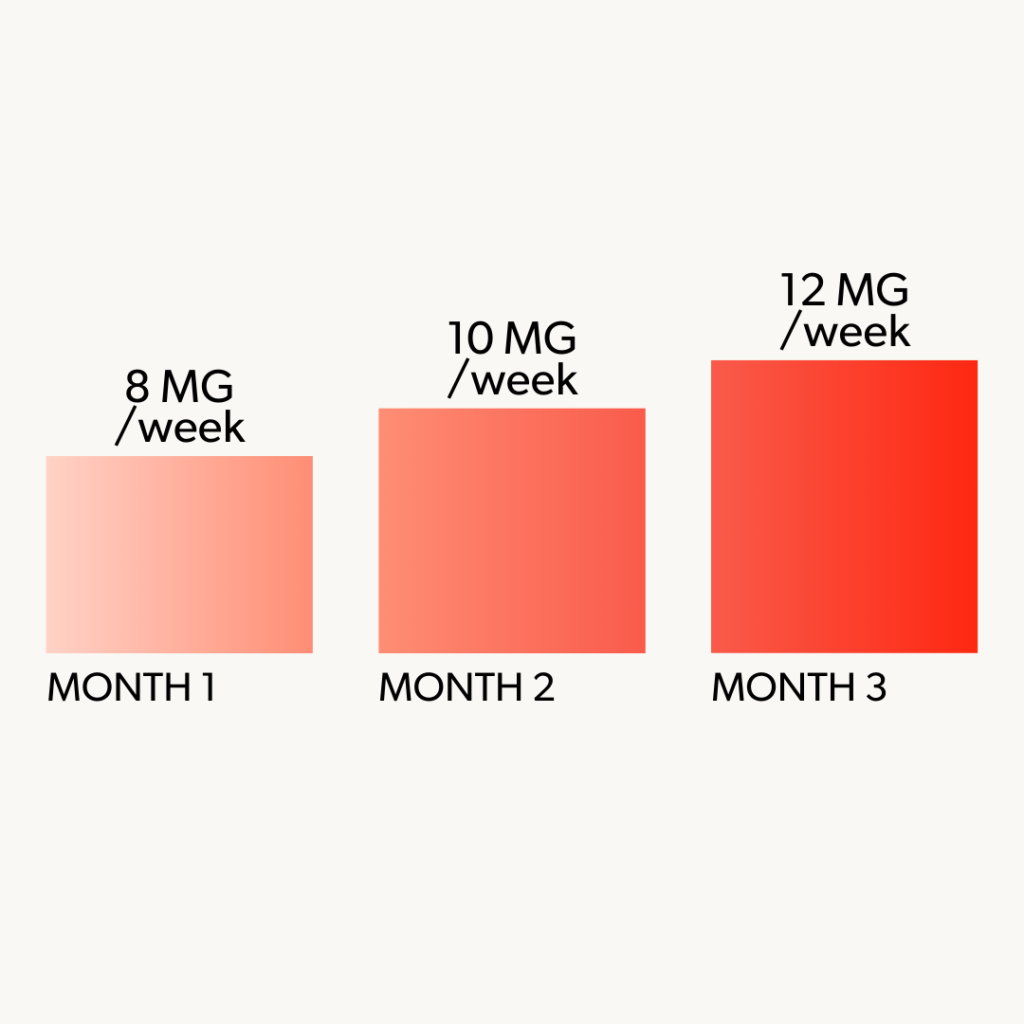
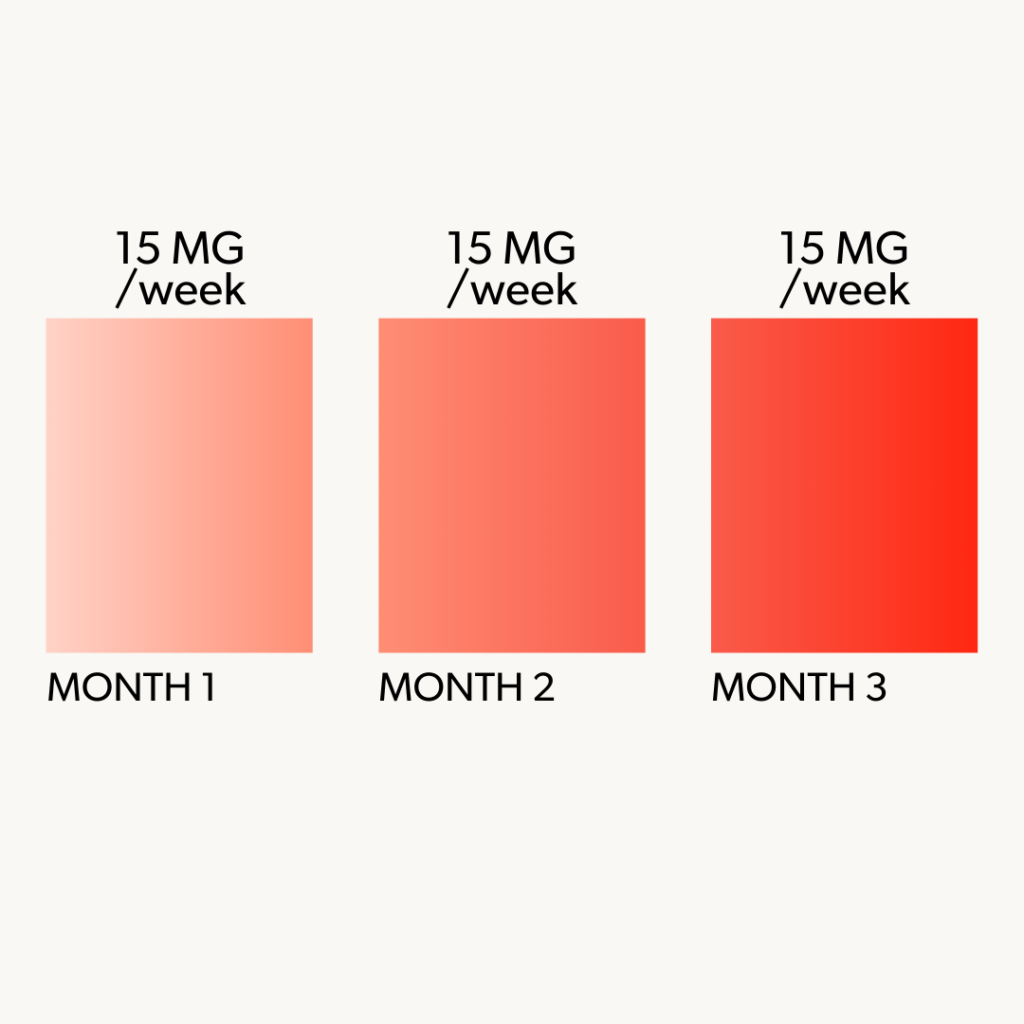
3 Month Minimum Requirement
Price includes doctor consultation and shipping fees.
Final pricing will vary depending on your personalized prescription and tier.
PLEASE NOTE: The following states are currently experiencing a temporary 10-15 day delay:
AL , AR, CA, HI, KS, MI, MN, MS
Tirzepatide is designed to support weight management and improve metabolic health. It is a dual-action treatment that targets both the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors, offering a comprehensive approach to weight loss and glucose control.
Prescription appetite suppression:
Tirzepatide curbs appetite and reduces food cravings, promoting weight loss.
Scientifically backed weight loss aid: Tirzepatide is an effective weight loss aid, as demonstrated in clinical trials where some patients shed up to 22.5% of their body weight over 6-8 weeks.
Metabolic health booster:
Tirzepatide improves metabolic health by lowering blood sugar levels, improving insulin sensitivity, and decreasing the risk of health problems that often accompany obesity such as type 2 diabetes, high blood pressure, and cardiovascular disease.
Enhanced quality of life:
Tirzepatide assists in reaching a healthy weight, which can reduce symptoms like fatigue and joint pain linked to excess weight. It also improves mobility and boosts energy levels.
Read what our customers have to say about our products.


Find answers to common questions about our weight loss products, including usage, ingredients, side effects and expected results.
Tirzepatide is an FDA-approved weekly injection used to treat type 2 diabetes. Although its primary purpose is for diabetes management, doctors may prescribe it off-label for weight loss. Clinical trials have demonstrated that tirzepatide can aid in weight loss, despite lacking FDA approval specifically for this purpose.
At EllieMD, our healthcare providers may prescribe a customized version of tirzepatide, potentially containing additional metabolic support ingredients. The pharmacy that ships your medication will depend on your state of residence, and each partnered pharmacy may offer its unique blend of metabolic support ingredients. As a result, you may receive different metabolic support ingredients based on your location.
Tirzepatide is generally safe when used as prescribed and monitored by a healthcare provider. However, like any medication, it can cause side effects. Common side effects of tirzepatide include nausea, vomiting, diarrhea, and constipation. These side effects are usually mild and resolve over time. In rare cases, more serious side effects like severe stomach or kidney problems may occur.
It’s important to discuss the potential risks and benefits of tirzepatide with your healthcare provider and to promptly report any side effects or concerns you may have.
Semaglutide is a medication approved by the FDA for treating type 2 diabetes and helping with long-term weight management. Tirzepatide is also FDA-approved for type 2 diabetes treatment. While it’s mainly used for diabetes, doctors may sometimes prescribe tirzepatide “off-label” to help with weight loss. Even though it hasn’t been officially approved for this purpose by the FDA, studies have shown that tirzepatide can help people lose weight.
Both semaglutide and tirzepatide are part of a group of drugs known as glucagon-like peptide-1 receptor agonists (GLP-1 RAs). They work by acting like a hormone called GLP-1, which helps control blood sugar levels. Tirzepatide goes a step further by also mimicking another hormone called glucose-dependent insulinotropic polypeptide (GIP). Research has found that the combined effect of GLP-1 and GIP in tirzepatide is more effective for weight loss compared to semaglutide.
To be eligible for our weight loss medication, you must meet certain requirements:
Your healthcare provider affiliated with EllieMD will make the final decision regarding your eligibility for the weight loss program and may request additional information directly from you.
Tirzepatide/Semaglutide may not be suitable for everyone.
The following conditions can prevent the use of semaglutide: personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, current use of another GLP-1 receptor agonist, diabetic ketoacidosis, known hypersensitivity to semaglutide or any of its injection components, recent bariatric surgery within the last 6 months, and pregnancy.
Additionally, it is important to inform your doctor if you have or have had a stomach or intestinal disorder, pancreatitis, kidney disease, or diabetes-induced eye problems.